BIO AMOVIR SAS
179 rue du Poirier
14650 CARPIQUET contact: contact@bioamovir.com
FRANCE
©2020 par BIO AMOVIR SAS.

Humasis COVID-19
Nasopharyngeal AG TEST
- Cost-effective, high performing test designed for
decentralized testing
- Simple test procedure using a direct
nasal swab
- Enable intuitive sample processing with prefilled,
utilized tubes
- Room Temperature Storage by 18 months
- Rapid results within 15 minutes without instrument
required

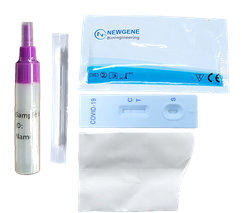
Novel Coronavirus Spike
Glycoprotein Salivary
Detection Kit
- This product is suitable for the qualitative detection
of novel coronavirus.
- It provides an aid in the diagnosis of infection with
novel coronavirus.
- Rapid detection of sputum and stool samples of
patients
- Package: 25 Tests/Kit
TESTS ANTIGÉNIQUES
TESTS SEROLOGIQUES
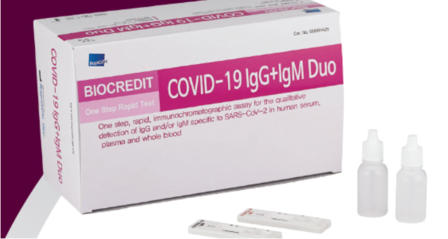
Biocredit COVID 19 IgM/IgG Rapid Test
Increases your COVID-19 Antibodies Testing Capacity
For patients with suspected active COVID-19 infection.
- The first commercialization Black Gold Particle technology
In the world (high sensitivity and specificity)
- User friendly Dual Color System (Control line: Red, Test
line: Black)
- Intended Use: Detection of SARS-CoV2 Antibodies
- Package: 25 Tests/Kit
- Storage: 1~40℃
- Specimen Type: Nasopharynx /Nasopharyngeal
- Shelf life: 12 months from manufacture date
- Time to result: 5~10 minutes
BIO AMOVIR SAS
179 rue du Poirier
14650 CARPIQUET
FRANCE contact: contact@bioamovir.com
©2020 par BIO AMOVIR SAS.


TESTS ANTIGÉNIQUES
Humasis COVID-19
nasopharyngeal AG TEST
- Cost-effective, high performing test
designed for decentralized testing
- Simple test procedure using a
direct nasal swab
- Enable intuitive sample processing with
prefilled, utilized tubes
- Room Temperature Storage by 18 months
- Rapid results within 15 minutes without
instrument required
Novel Coronavirus Spike Glycoprotein
Salivary Detection Kit
- This product is suitable for the qualitative
detection of novel coronavirus.
- It provides an aid in the diagnosis of infection
with novel coronavirus.
- Rapid detection of sputum and stool samples
of patients
- Package: 25 Tests/Kit
TESTS SEROLOGIQUES
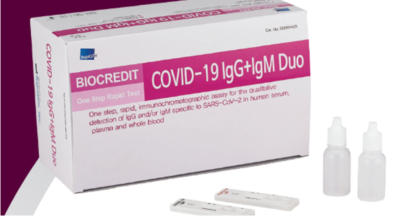
Biocredit COVID 19 IgM/IgG Rapid Test
Increases your COVID-19 Antibodies Testing
Capacity For patients with suspected active COVID-
19 infection.
- The first commercialization Black Gold
Particle technology In the world (high
sensitivity and specificity)
- User friendly Dual Color System (Control
line: Red, Test line: Black)
- Intended Use: Detection of SARS-CoV2
Antibodies
- Package: 25 Tests/Kit
- Storage: 1~40℃
- Specimen Type: Nasopharynx
/Nasopharyngeal
- Shelf life: 12 months from manufacture date
- Time to result: 5~10 minutes